The largest international study ever conducted on Alzheimer’s disease (AD), the I-GAP (International Genomics Alzheimer’s Project) consortium has identified 11 new regions of the genome involved in the onset of this neurodegenerative disease.
This study gives an overview of the molecular mechanisms underlying the disease, opening up to a better understanding of the pathophysiology of AD. These results detailed currently in Nature Genetics, could not have been obtained without this unique worldwide collaborative effort.
Since 2009, 10 genes for Alzheimer’s disease have been identified. However, much of the individual susceptibility to develop the disease remains unexplained. So in February 2011, the leaders of the four largest international research consortia on the genetics of AD joined forces to accelerate the discovery of new genes. Supported in part by the National Institute on Aging (NIA) and other components of the National Institutes of Health (NIH), in less than three years, the IGAP program identified more genes than had been identified over the previous 20 years. They collected genetic data on 74,076 patients and controls from 15 countries and were able to discover 11 new genes in addition to those already known, and identify 13 other genes, yet to be validated.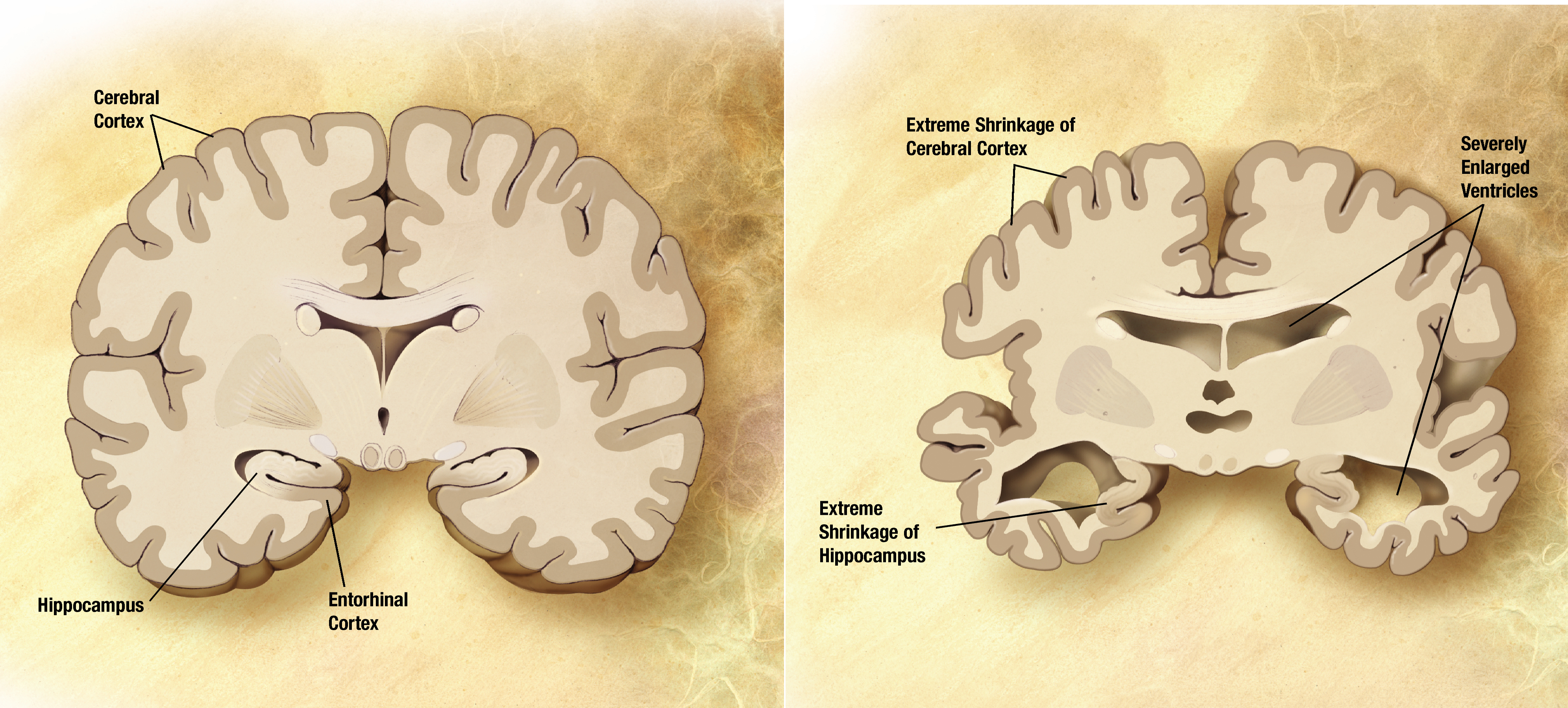
These 11 new confirmed genes may open new avenues to understanding the causes of AD. For example, one of the most significant associations was found in the region HLA-DRB5/DRB1 major histocompatibility complex. This finding is interesting in several ways. First, it strongly suggests the involvement of the immune system in AD. In addition, this same region has also been associated with two other neurodegenerative diseases, one known to have an immune mechanism, multiple sclerosis and another not previously thought to have a major immune component, Parkinson’s disease.
Some of the newly associated genes confirm biological pathways known to be involved in AD, including the amyloid (SORL1, CASS4 ) and tau (CASS4 , FERMT2 ) pathways. The role of the immune response and inflammation (HLA-DRB5/DRB1 , INPP5D , MEF2C ) already implied by previous work (CR1, TREM2) is reinforced, as are the importance of cell migration (PTK2B), lipid transport and endocytosis (SORL1 ). New hypotheses have also emerged related to hippocampal synaptic function (MEF2C , PTK2B), the cytoskeleton and axonal transport (CELF1 , NME8, CASS4) as well as myeloid and microglial cell functions (INPP5D).
Finally, this work demonstrates that, given the complexity of such a disease, only a global collaboration of research efforts will quickly find solutions to tackle this major threat.
The four founding partners in this international consortium are, in alphabetical order, the Alzheimer’s Disease Genetics Consortium (ADGC), the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE), the European Alzheimer Disease Initiative (EADI) and the Genetic and Environmental Research in Alzheimer Disease (GERAD) consortium.
Boston University and the Framingham Heart Study are well-represented in this landmark international effort. The neurology working group of the Cohorts for Heart and Aging Research in Genomic Epidemiology is led by Sudha Seshadri, MD, professor of neurology at Boston University School of Medicine (BUSM), who is a senior investigator in the Framingham Heart Study and also one of the senior authors on this manuscript. Several other senior investigators, notably Anita L. DeStefano , PhD, professor of biostatistics Boston University School of Public Health (BUSPH) on behalf of CHARGE, and Lindsay A. Farrer, PhD, Chief of Biomedical Genetics and professor of medicine, neurology, ophthalmology, genetics & genomics, epidemiology, and biostatistics at BUSM and BUSPH on behalf of the ADGC, were key investigators in this effort. Farrer co-directs the data analysis effort for the ADGC which includes nearly all of the nation’s researchers working on the genetics of AD as well as many investigators and resources of the 29 NIA funded Alzheimer Disease Centers.
“This study clearly demonstrates that there really is strength in numbers to identify genes that individually have a small effect on risk of Alzheimer’s,” said Farrer. “But it’s not the magnitude of the odds ratio that’s really important. Each gene we implicate in the disease process adds new knowledge to our understanding of disease mechanism and provides insight into developing new therapeutic approaches, and ultimately these approaches may be more effective in halting the disease since genes are expressed long before clinical symptoms appear and brain damage occurs,” he added.
“This landmark international effort has uncovered new pathways and new genes in old pathways that are definitely associated with Alzheimer dementia, but we need to do much work to better understand how exactly these genes work in health and disease, and to perhaps make drugs from these genes and molecules,” said Seshadri. “We will continue to mine these results for new insights, even as we include more patients and use new technologies like whole genome sequencing to find more new pathways and genes,” she added.

